Hernia Mesh Mass Torts Lawsuits

“What Do I Need To Know About Hernia Mesh Mass Torts?”
Hernia mesh mass torts are based on complications following mesh implant surgery. These include constipation, chronic pain, sexual dysfunction and even diarrhea. Hernia mesh lawsuits are generally based on the theory that the implants were defectively designed. Failure to warn of the defects resulted in patients being exposed to foreseeable injuries. There are thousands of hernia mesh lawsuits pending against implant manufacturers as of this writing.
The Kann California Law Group now offers Hernia Mesh Mass Tort representation. Unlike criminal law, which is concerned primarily with incarceration, hernia mesh implant injury law focuses on compensation for pain and remuneration for lost earnings. Below you'll find a definition of hernia, facts about how hernias are repaired, information on which complications are being reported owing to defective hernia mesh implants, symptoms associated commonly with defective hernia mesh implants, a list of companies producing defective hernia mesh implants, insight into the FDA's monitoring process and what a product recall can mean for your case, help on grounds for filing a hernia mesh implant lawsuit, facts about damages available in hernia mesh mass torts, a statement about how mass torts allow suit outside a class action, an important consideration about how much you should expect from a settlement, and what you should do if you've been injured by a defective hernia mesh implant.
Please feel free to contact the Kann California Law Group in Santa Clarita, Ventura, Encino, Pasadena or Los Angeles/Los Angeles County with further questions. Your call goes directly to a lawyer. Guaranteed.
“What Is A Hernia?”

A hernia is a sac formed by the lining of the abdomen. The sac comes through a layer of the belly called fascia that surrounds the muscle. This forms a lump called a hernia.[1] Hernias can be acquired or they can be native traits. Typically, hernias are suffered owing to lifting heavy objects or repeated coughing.
Hernias of the groin make up approximately 75% of hernia cases. Surprisingly, groin hernias exist in only three different subtypes. However, abdominal hernias, which make up the remaining quarter of hernia injuries, may be found in several different forms.
“How Are Hernias Repaired?”
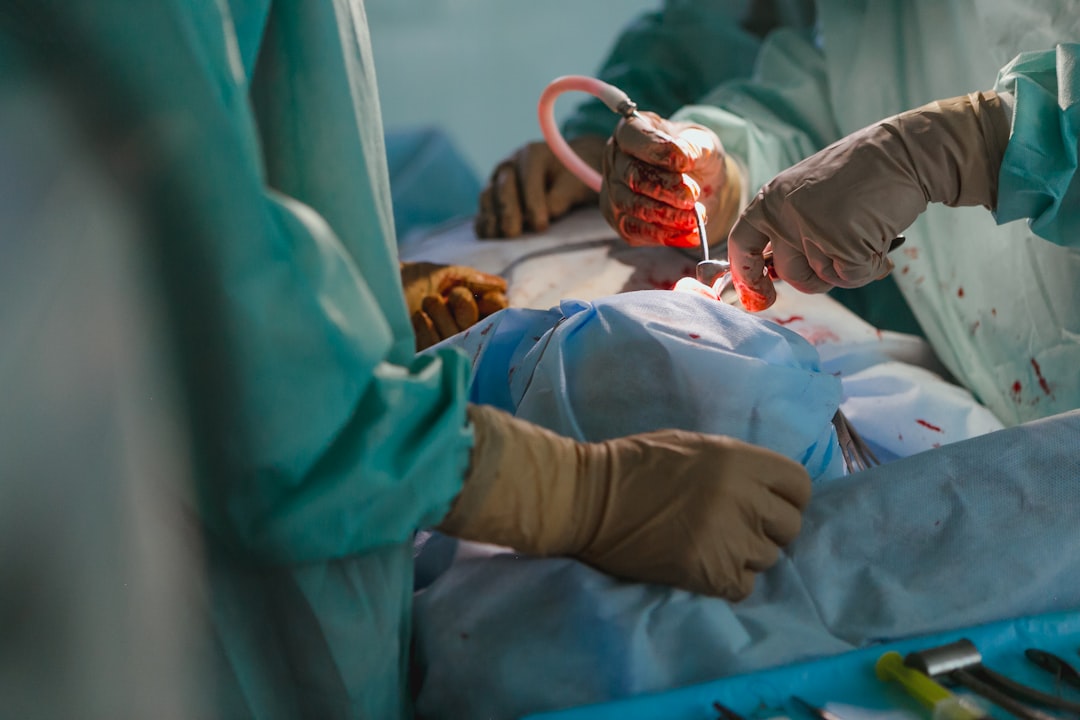
Hernias are repaired using the patient's own (“native”) tissues and sutures most of the time. Mesh is employed only if the native tissue approach is ineffective. Within the patient, some mesh implants are overlaid, some are inlaid, and some are underlaid.
“What Complications Are Being Reported Owing To Defective Hernia Mesh Implants?”
The following complications are being reported owing to defective hernia mesh implants.
Note: This list is not exhaustive.
Cancer: It is possible for cancer to develop from an infected hernia mesh implant.
Chronic Pain: Often in connective tissues and joints. A symptom of several other complications.
Mesh Failure: Total failure of the hernia mesh device may require removal of the implant.
Groin/Testicular Pain: Groin/testicular pain may necessitate testicle removal.
Infection: Has been known to occur more than a month after surgery. Often associated with fever, sweating, and sepsis.
Seromas: Infections following fluid accumulation under the surface of the skin.
Recurrence Of Hernia: Recurrence often requires another surgical procedure.
Abdominal Pain: Often accompanies nausea. May indicate nerve damage, infection, or internal injury.
Bowel Obstruction: Occurs when mesh migration and/or adhesion to tissue prevents use of the bowels.
Adhesion: The circumstance in which a hernia mesh implant sticks to internal tissue of some kind.
Migration: Involves the hernia mesh implant moving from its implantation site.
Perforation Of Tissues/Organs: A symptom of hernia mesh implant erosion, shrinkage, or migration.
Erosion Of Implant Device: Erosion can cause infection, pain, nausea and/or fever.
“Are There Symptoms Associated Commonly With A Defective Hernia Mesh Implant?”
There are symptoms associated commonly with a defective mesh hernia implant. Here's a brief list:
1) Chills;
2) Fever;
3) Swelling;
4) Bruising;
5) Difficulty urinating;
6) Infection; and,
7) Erectile dysfunction.
“Which Companies Are Producing Defective Hernia Mesh Implants?”

Below is a list of companies which are producing defective mesh implant claims in the greatest number.
Remember: This is not a comprehensive list of mesh devices or of potentially liable mesh manufacturers.
Atrium Medical: Manufacturer of ten types of hernia mesh products. This includes C-QUR hernia mesh, which caused injuries to many. Atrium products include TacShield, C-QUR Mosaic and C-QUR V-Patch.
Covidien: Parietex mesh is Covidien's best-known product. Other Covidien implants include Parietex Composite, Parietex ProGrip, and Parietex Plug & Patch System.
C.R. Bard: Two of the largest mass tort settlements in a hernia mesh implant case involved C.R. Bard, a company that creates its own mesh coating using polypropylene, a substance which harms patients.
Ethicon: Ethicon Physiomesh has been recalled in Europe and was the subject of a market withdrawal in this country. Physiomesh complications have been reported by many because of the coating Monocryl.[2]
Gore Medical: Gore Medical is best known for Gore-Tex, a mesh which has fallen out of popular favor. Gore makes medical devices with its Gore-Tex. As a result, these devices can injure implant patients.
Genzyme Corporation: Genzyme created Sepramesh IF. This device has caused severe complications. Both Genzyme and Bard now manufacture Sepramesh IP, which may have cause complications as well.
“Doesn't The Food & Drug Administration Monitor Mesh Implants?”

Yes, the FDA monitors mesh implants.[3]
The FDA must approve all medical devices on the market and monitor safety. However, since the FDA uses the 510(k) clearance process for hernia mesh implants,[4] manufacturers need only show that there's an equivalent device on the market to their own devices, and that, compared to the marketed devices, their new devices have the same intended uses and technological characteristics or the same intended uses but different technological characteristics. As a result, the 510(k) clearance process allows the use of untested devices in surgical procedures. Furthermore, once a defective hernia mesh implant passes normal FDA muster, the 510(k) clearance process quickly ushers in all similar defective mesh implants.
“How Does The Food & Drug Administration Monitor Hernia Mesh Implants?”
The FDA monitors hernia mesh implants by reviewing injury reports and post-market events. The Administration issues a warning letter when it is clear that a device has issues, permitting review of the issue to proceed. If a problem is subsequently found, the device is recalled. But recalls can be voluntary or mandatory; the facts of the situation and the device will dictate. If the problem isn't corrected, or can't be remedied, the device will be permanently recalled and its sale will be completely prohibited.[5]
“Could My Hernia Mesh Implant Being Recalled Affect My Case?”
Yes, your hernia mesh implant being recalled could affect your case.
Hernia mesh implants are often approved without careful testing. In fact, testing does not guarantee reliability. Some thoroughly vetted meshes turn out defective when used. Therefore, when hernia mesh is recalled, the product recall may serve as evidence of the mesh's defects. When this happens, proof of a manufacturer's liability becomes as simple as connecting the defect to your injury. This is a significant help in proving that a hernia mesh implant was defective and caused an injury or a complication.
Grounds For Filing A Hernia Mesh Implant Lawsuit

Grounds for filing hernia mesh lawsuit include:
Design Defects
Design defects make products inherently dangerous, as defective designs cannot be made safe through improving the quality of manufacturing or the labeling of any product. Defectively designed hernia mesh implants are thus inherently dangerous.
Hernia mesh manufacturers are held to a “strict liability” standard.[6] They become for injuries if: 1) A defective design posed a foreseeable risk; and, 2) The mesh was manufactured as intended; and, 3) The mesh was used as intended. No further proof of causing an injury is required.
Defective Labeling/Failure To Warn
Defective labeling involves hernia mesh manufacturers failing to disclose risks so that doctors and patients could be fully informed of the risks of using their implants. The law requires: 1) That physicians be warned of non-obvious dangers mesh may cause; and, 2) That physicians be informed of how to avoid or limit the risks.
Failure to warn[7] of risks, on the other hand, requires showing: 1) That mesh packaging and/or instructions omitted warnings; and, 2) There was reliance on the warning by doctors and/or patients.
Medical Malpractice
Medical malpractice requires showing that a doctor engaged in professional negligence when providing medical services. The pleading must show: 1) That the doctor owed a duty of care to the patient; 2) That the doctor breached the duty through professional negligence; and, 3) That the breach caused an injury.
Manufacturing Defects
Manufacturing defects involve deviation from an original design and/or intended materials in producing something. These defects can be especially dangerous. To prove a manufacturing defect, your lawsuit must demonstrate: 1) That your hernia mesh had a manufacturing defect at the time it left the manufacturer's possession; and, 2) That the defect was a substantial factor in causing your injury.
“What Damages I Can Sue For In A Hernia Mesh Implant Lawsuit?”
You can sue for damages known generally as “economic” and “non-economic damages” in a hernia mesh implant lawsuit.
Economic Damages
Economic damages commonly include medical bills, lost wages, and out-of-pocket expenses like costs associated with attending medical appointments. Additionally, expenses like future wage losses and future medical costs can be awarded (with proof of proper facts). There is no cap on economic damages. The total sum of your economic damages will, therefore, equal the total compensation owed to you.
Non-Economic Damages
Non-economic damages compensate for pain and suffering. This includes physical pain, whether originating with the mesh or any subsequent surgical procedure necessary to address complications. Note, however, that mental and emotional suffering is also compensable if you suffer mental or emotional damage because of your mesh injury.
There is no set formula for compensating people claiming non-economic damages. Relevant factors include: The length of time recovery your is expected to require; how the injury affects your day-to-day activities; your prognosis; the type of injury; the severity of your injury; whether your injury will produce permanent disability or disfigurement; and how your injury will affect your personal relationships. Thus, spouses may also file claims based on loss of consortium, a form of non-economic damage award arising from an injury that directly exerts a negative impact on a marriage, such as loss of intimate relations.
There are state caps on non-economic damages. But not all states have such damage limitations. The states that do have caps create differing upper limits, depending on whether a tort case is in personal injury, product liability, or a form of medical malpractice.[8]
Punitive Damages
Punitive damages punish defendants. They can be quite substantial. As a result, most settlements don't include them. However, punitive damages are relevant in mass torts. Judges may impose these damages on mesh manufacturers where the manufacturers engage in egregious behavior like intentionally failing to disclose a known risk associated with a hernia mesh implant.
Forms Of Damage Award: In Brief
Medical Costs: An award that may include the cost of long-term hospital care, the expenses associated with physical or occupational therapy, prescription/drug costs, and other expenses.
Loss of Earnings/Wages: An award creating compensation for lost wages because injury associated with a hernia mesh implant caused you to miss work.
Loss of Earning Capacity: An award compensating you for the loss of the ability to earn money because injury associated with a hernia mesh implant caused you to lose earning capacity.
Pain and Suffering Compensation: Amounts compensating you for physical pain, mental suffering, loss of enjoyment of life, disfigurement, physical impairment, anxiety, and/or emotional distress.
“Will I Have To Sue As Part Of A Class Action, Or Can I Bring My Own Lawsuit For A Hernia Mesh Implant Injury?”
You can maintain your own lawsuit for a hernia mesh implant injury.
While plaintiffs band together to sue as one in a class action, individual lawsuits may be filed against a single defendant in what is known as a mass tort action.[9] In federal court, this is known as multidistrict litigation (MDL). It is the process of consolidating diverse cases into one for pretrial purposes.[10] There are also multi-county litigations (MCLs). These approximate MDLs, but on the state court level. As mass torts, MCLs also permit individual suit instead of class action.
“How Much Should I Expect To Receive In My Hernia Mesh Mass Tort Settlement?”
No prediction can be made about your hernia mesh mass tort settlement amount. A handful of MDL plaintiffs are proceeding with their claims in what are being termed “bellwether trials.” High damage awards to these plaintiffs might generate higher value settlements for the remaining plaintiffs - but this process could take years.
Furthermore, the value of your settlement may be affected by whether a final settlement is “global” or “inventory.” Global settlements involve payment of all plaintiffs at the same time. Inventory settlements involve a manufacturer reaching an individual settlement deal with the law firms representing plaintiffs. If a manufacturer faces an inventory settlement, it might go to firms that have the most cases and try to establish a market pattern using those settlements – all before you have the opportunity to seek more.
“What Should I Do If I've Been Injured By A Hernia Mesh Implant?”
If you've been injured by a hernia mesh implant, see a doctor. Medical attention is always advisable when it comes to an internal medical device. This is particularly true if the symptoms of illness or injury persist for longer than thirty days (one month) after implantation. Remember: Hernia mesh failure and resulting injuries can manifest in several ways. So, tell your doctor about your hernia mesh implantation.
Then: Make sure you file claims within the statute of limitations duration. California gives you one year from the point you discovered your injury (or should've discovered your injury) to file your claim.[11] If you fail to file within this time limit, you can lose your eligibility to file a lawsuit altogether. Contact the Kann California Law Group attorneys as soon as you suspect you've been injured by your hernia mesh implant.
Contact the Kann California Law Group

The State of California regards Hernia Mesh Implant lawsuits as serious concerns. If you're involved in a Hernia Mesh Mass Tort, it's essential you retain a skilled, dedicated attorney as soon as possible. Your rights and livelihood may be at stake.
Remember, a professional Hernia Mesh Mass Tort law attorney may be able to:
- Negotiate an award;
- Win your case at trial;
- Or get other legal relief for you, as appropriate.
The attorneys at the Kann California Law Group have an excellent understanding of the local courts and an extensive knowledge of California's personal injury justice system. We can represent you in Ventura, Santa Clarita, Los Angeles, Encino, Pasadena, Kern, San Bernardino, Orange, Santa Barbara, and Riverside Counties, and many other Southern California counties and cities.
If you or someone you know is suing for a hernia mesh implant injury, our attorneys will evaluate the facts of the case and plan a strategy that will help to obtain the best possible outcome.
Contact the Kann California Law Group today to schedule your free and confidential consultation. Use our confidential contact form or call us at 888-744-7730.
[1] See “Hernia” at Medlineplus.org.
[2] So frequent became the complaints that the Physiomesh product is subject to a specific order establishing its future preservation and handling. See "In Re: Physiomesh Flexible Composite Hernia Mesh Products Liability Litigation," Civil Action No. L17-MD-02782-RWS (SD Ga. 2018)."
[3] See “General Controls for Medical Devices,” FDA.gov, March 22, 2018.
[4] The 510(k) clearance process is one of two principal review processes, Premarket Approval being the other.
[5] See “Recalls, Market Withdrawals and Safety Alerts,” FDA.gov.
[6] See “Strict Liability” at USLegal.com.
[7] “The first duty for any product manufacturer is to use reasonable care in designing and making a product so that it is not unreasonably dangerous. Because a product that is not unreasonably dangerous may still have some dangerous features or functions, a manufacturer must also provide with the product warnings that identify the dangerous aspects of the product. An adequate warning mitigates the risk of harm posed by a product because it allows consumers to make informed choices about whether and how to encounter certain risks.” See “Understanding A Medical Device Manufacturer's Broad Duty to Warn” by Terry M. Henry, Melanie S. Carter, Lauren E. O'Donnell, and Naomi Zwillenberg. MDDIOnline.com, April 14, 2017.
[8] See “Damage Caps in California Personal Injury Claims” by Dan Kann. Kannlawoffice.com, September 18, 2021.
[9] See “Mass Tort Law and Legal Definition” at USLegal.com.
[10] See 28 USC [United States Code] § [Section] 1407, Ch. 87, Part IV. Govregs.com.






